Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Satoru; Sato, Haruo; Ishidera, Takamitsu; Fujii, Naoki*; Kawamura, Katsuyuki*
JNC TN8400 2001-031, 44 Pages, 2002/05
In order to quantify effect of temperature on diffusivity of deuterated water (HDO) in compacted sodium-bentonite, through-diffusion experiments were conducted at elevated tempemture from 298 to 333 K. Kunipia F (Na-montmorillonite content  98 wt. %; Kunimine Industly Co.) was compacted to a dry density of 0.9 and l.35 Mg/m
98 wt. %; Kunimine Industly Co.) was compacted to a dry density of 0.9 and l.35 Mg/m . Since smectite flakes were perpendicularly oriented to a direction of compaction, anisotropy of diffusivity was investigated parallel and normal to the preferred orientation of smectite. Effective diffusion coeficient D
. Since smectite flakes were perpendicularly oriented to a direction of compaction, anisotropy of diffusivity was investigated parallel and normal to the preferred orientation of smectite. Effective diffusion coeficient D of HDO was larger for a diffusional direction parallel to the preferred orientation than normal to that for both dry densities. These results well agreed to the previously reported ones for tritiated water. Activation energies of D
of HDO was larger for a diffusional direction parallel to the preferred orientation than normal to that for both dry densities. These results well agreed to the previously reported ones for tritiated water. Activation energies of D in compacted bentonite increased with increasing dry density in the range of 19 - 25 kJ/mol which was slightly larger than that in bulk water (18 kJ/mol). This relationship can be considered to be due to both the pore structure development and high activation energy of water (18-23 kJ/mol) in the vicinity of smectite surface (within 2 nm) on the basis of molecular dynamics simulations.
in compacted bentonite increased with increasing dry density in the range of 19 - 25 kJ/mol which was slightly larger than that in bulk water (18 kJ/mol). This relationship can be considered to be due to both the pore structure development and high activation energy of water (18-23 kJ/mol) in the vicinity of smectite surface (within 2 nm) on the basis of molecular dynamics simulations.
Taniguchi, Naoki; Kawakami, Susumu; *
JNC TN8400 2001-025, 27 Pages, 2002/03
It is essential to understand the corrosion type of carbon steel under the repository conditions for the lifetime assessment of carbon steel overpack used for geological isolation of high-level radioactive waste. According to the previous study, carbon steel is hard to passivate in buffer material assuming a chemical condition range of groundwater in Japan. However, concrete support will be constructed around the overpack in the case of repository in the soft rock system and groundwater having a higher pH may infiltrate to buffer material. There is a possibility that the corrosion type of carbon steel will be influenced by the rise of the pH in groundwater. In this study, anodic polarization experiments were performed to understand the passivation condition of carbon steel in buffer material saturated with water contacted with concrete. An ordinary concrete and a low-alkalinity concrete were used in the experiment. The results of the experiments showed that the carbon steel can passivate under the condition that water having pH  13 infiltrate to the buffer material assuming present property of buffer material. If the low-alkalinity concrete is selected as the support material, passivation can not occur on carbon steel overpack. The effect of the factors of buffer material such as dry density and mixing ratio of sand on the passivation of carbon steel was also studied. The results of the study showed that the present property of buffer material is enough to prevent passivation of carbon steel.
13 infiltrate to the buffer material assuming present property of buffer material. If the low-alkalinity concrete is selected as the support material, passivation can not occur on carbon steel overpack. The effect of the factors of buffer material such as dry density and mixing ratio of sand on the passivation of carbon steel was also studied. The results of the study showed that the present property of buffer material is enough to prevent passivation of carbon steel.
JNC TN8400 2000-012, 33 Pages, 2000/04
The redox condition of near-field is expected to affect the performance of engineered barrier system. Especially, the oxygen initially existing in the pore space of compacted bentonites strongly affects the redox condition of the near-field. For assessing the influence of the oxygen, the transport parameters of it in the compacted bentonite and consumption process should be known. Therefore, following researches were conducted. In order to understand the diffusion of dissolved oxygen (DO) in compacted bentonite and to predict the effect of DO, the effective diffusion coefficients of DO in compacted sodium bentonite were measured by electrochemistry. As the results, the following relationship between the dry density of compacted sodium bentonite and the effective diffusion coefficient of DO in compacted sodium bentonite was derived: De=1.53 0.13
0.13 10
10 exp(-2.15
exp(-2.15 0.24
0.24 10
10 p) where De is the effective diffusion coefficient (m
p) where De is the effective diffusion coefficient (m s
s ) of DO in compacted sodium bentonite and
) of DO in compacted sodium bentonite and  is the dry density (kg m
is the dry density (kg m ) of compacted sodium bentonite. The oxygen concentration in the bentonite is expected to be controlled by oxidation of pyrite as impurity in the bentonite. In order to investigate the above idea, the rates of pyrite oxidation by DO in compacted sodium bentonite were estimated from the experimental data on pyrite-bentonite systems usig the obtained effective diffusion coefficient of DO. The results show that the averages of the rate constants of pyrite oxidation by DO in the bentonite for dry densities of 0.8, 0.9, 1.0, 1.1 and 1.2
) of compacted sodium bentonite. The oxygen concentration in the bentonite is expected to be controlled by oxidation of pyrite as impurity in the bentonite. In order to investigate the above idea, the rates of pyrite oxidation by DO in compacted sodium bentonite were estimated from the experimental data on pyrite-bentonite systems usig the obtained effective diffusion coefficient of DO. The results show that the averages of the rate constants of pyrite oxidation by DO in the bentonite for dry densities of 0.8, 0.9, 1.0, 1.1 and 1.2 10
10 kgm
kgm were 1.38
were 1.38 0.32
0.32 10
10 , 1.10
, 1.10 0.24
0.24 10
10 , 1.16
, 1.16 0.35
0.35 10
10 , 9.36
, 9.36 2.23
2.23 10
10 and 7.48
and 7.48 1.92
1.92 10
10 ms
ms , respectively. The relationship between the dry density (
, respectively. The relationship between the dry density ( ) and the rate constant (k') was expressed as follows: k'=3.94
) and the rate constant (k') was expressed as follows: k'=3.94 1.06
1.06 10
10 exp(-1.33
exp(-1.33 0.28
0.28 10
10
 ) ...
) ...
*; *; *
JNC TJ8400 2000-018, 79 Pages, 2000/02
As a basic research for geological disposal of high-level radioactive wastes, diffusion behavior of radionuclides and corrosion behavior of overpack materials in clay buffer materials (bentonite) were studied. In the study on the diffusion behavior of radionuclides, basal spacing and water content were determined for water saturated, compacted Na-montmorillonite that is major clay mineral of bentonite. The apparent diffusion coefficients of Na , Sr
, Sr , Cs
, Cs and Cl
and Cl ions and their activation energies were also determined at different dry densities of montmorillonite. For all kinds of ions, the activation energies were found to increase as the dry density increased. These findings suggest that the diffusion mechanism of ions in compacted montmorillonite changed with increasing dry density. As a reasonable explanation for the changes in the activation energy, we proposed a multiprocess diffusion model, in which predominant diffusion process is considered to change from pore water diffusion to interlayer diffusion via surface diffusion when the dry density increases. The Na-montmorillonite is expected to alter by the ion exchange with Ca
ions and their activation energies were also determined at different dry densities of montmorillonite. For all kinds of ions, the activation energies were found to increase as the dry density increased. These findings suggest that the diffusion mechanism of ions in compacted montmorillonite changed with increasing dry density. As a reasonable explanation for the changes in the activation energy, we proposed a multiprocess diffusion model, in which predominant diffusion process is considered to change from pore water diffusion to interlayer diffusion via surface diffusion when the dry density increases. The Na-montmorillonite is expected to alter by the ion exchange with Ca ions, which could be introduced from groundwater and/or cementitious materials in a repository. The apparent diffusion coefficients of Na
ions, which could be introduced from groundwater and/or cementitious materials in a repository. The apparent diffusion coefficients of Na and Cs
and Cs ions and their activation energies were studied for Na/Ca montmorillonite mixtures in order to know the effect of this kind of alteration on the diffusion behavior of ions. It was found that the alteration of montmorillonite affected diffusion coefficients and the activation energies for both kinds of cations. These effects cannot be explained only by the pore water diffusion. The multiprocess diffusion model proposed in this study is suggested as the most reasonable explanation for the effects. The oxidation behavior of pyrite in bentonite during drying process was studied for understanding corrosion behavior of overpack materials in bentonite. There ...
ions and their activation energies were studied for Na/Ca montmorillonite mixtures in order to know the effect of this kind of alteration on the diffusion behavior of ions. It was found that the alteration of montmorillonite affected diffusion coefficients and the activation energies for both kinds of cations. These effects cannot be explained only by the pore water diffusion. The multiprocess diffusion model proposed in this study is suggested as the most reasonable explanation for the effects. The oxidation behavior of pyrite in bentonite during drying process was studied for understanding corrosion behavior of overpack materials in bentonite. There ...
Sugino, Hiroyuki; Fujita, Tomoo; Taniguchi, Wataru; Iwasa, Kengo; Hasegawa, Hiroshi
JNC TN8400 99-096, 23 Pages, 1999/12
The Japan Nuclear Cycle Development Institute (JNC) has prepared a second progress report (entitled H12) on research and development for geological disposal of high-level waste (HLW) in Japan. H12 report consist of a Project Overview Report and three Supporting Reports which cover the three major fields described in the AEC Guidelines: (1)evaluation of the geological environment, (2)repository design and engineering technology, (3)performance assessment. This report is prepared to explain background information of buffer design which is descried in Supporting Report 2 (Repository Design and Engineering Technology). In buffer design of H12 report, the design requirements of the buffer are assumed and the relationship between buffer thickness and density was shown corresponding design requirement as an area map. This report describes the background information such as the numerical formulations, assumptions, engineering judgement and so on.
Mihara, Morihiro; ; Ueta, Shinzo*; *
JNC TN8430 99-011, 27 Pages, 1999/11
In radioactive waste disposal, compacted Na-bentonite has been proposed for a buffer material. However, Na-bentonite would change to Ca-bentonite in the long term period. The change of Na-bentonite to Ca-bentonite might cause the change in the data concerning with nuclides migration properties such as permeability, sorption and diffusion. In this study, effective diffusion coefficients of HTO, Cs, I and C in compacted Ca-bentonite which was changed from Na-bentonite, Kunigel V1, were obtained and were compared to published those of Kunigel V1. In addition, effective diffusion coefficients for compacted Ca-bentonite with syncetic sea system water, SW, were obtained in order to investigate effect of solution composition. The magnitude of effective diffusion coefficients in Ca-bentonite are arranged in smaller order as Cs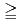 HTO
HTO I
I C. It is estimated that their effective diffusion coefficients are same those of Na-bentonite. About effect of solution composition, effective diffusion coefficients of HTO in 1.8g/cm
C. It is estimated that their effective diffusion coefficients are same those of Na-bentonite. About effect of solution composition, effective diffusion coefficients of HTO in 1.8g/cm dry density with SW were almost same values with distilled system water, DW. However, effective diffusion coefficients of HTO in lower density were smaller than values with DW. Regarding as effective diffusion coefficients of Cs in 1.8g/cm
dry density with SW were almost same values with distilled system water, DW. However, effective diffusion coefficients of HTO in lower density were smaller than values with DW. Regarding as effective diffusion coefficients of Cs in 1.8g/cm dry density, the effect of SW could not be observed as well as HTO. However, effective diffusion coefficients of I and C existing as an anion in pore water of bentonite increased by the reduction in the ion exclusion.
dry density, the effect of SW could not be observed as well as HTO. However, effective diffusion coefficients of I and C existing as an anion in pore water of bentonite increased by the reduction in the ion exclusion.
*; Nakazawa, Toshiyuki*; Ueta, Shinzo*; Shibata, Masahiro
JNC TN8400 99-069, 41 Pages, 1999/11
As a part of the evaluation for the sorption phenomena of nuclides in compacted bentonite, apparent diffusivities for uranium, neptunium and technetium that are redox-sensitive elements, were measured under reducing conditions. Bentonite used was a sodium bentonite, Kunigel V1. Apparent diffusivities were measured by using in-diffusion method (concentration profile method), under the conditions with varying dry densities of compacted bentonite and sorts of the solution used for water saturation of bentonite in diffusion experiments. As a result of the measurements, following ranges of values for apparent diffusivities were acquired. ...
Takachi, Kazuhiko; Suzuki, Hideaki*
JNC TN8400 99-041, 76 Pages, 1999/11
The buffer material is expected to maintain its low water permeability, self-sealing properties, radionuclides adsorption and retardation properties, thermal conductivity, chemical buffering properties, overpack supporting properties, stress buffering properties, etc. over a long period of time. Natural clay is mentioned as a material that can relatively satisfy above. Among the kinds of natural clay, bentonite when compacted is superior because (1)it has exceptionally low water permeability and properties to control the movement of water in buffer, (2)it fills void spaces in the buffer and fractures in the host rock as it swells upon water uptake, (3)it has the ability to exchange cations and to adsorb cationic radioelements. In order to confirm these functions for the purpose of safety assessment, it is necessary to evaluate buffer properties through laboratory tests and engineering-scale tests, and to make assessments based on the ranges in the data obtained. This report describes the procedures, test conditions, results and examinations on the buffer material of unconfined compression tests, one-dimensional consolidation tests, consolidated-undrained triaxial compression tests and consolidated-undrained triaxial creep tests that aim at getting hold of static mechanical properties. We can get hold of the relationship between the dry density and tensile stress etc. by Brazillian tests, between the dry density and unconfined compressive strength etc. by unconfined compression tests, between the consolidation stress and void ratio etc. by one-dimensional consolidation tests, the stress pass of each effective confining pressure etc. by consolidated-undrained triaxial compression tests and the axial strain rate with time of each axial stress etc. by consolidated-undrained triaxial creep tests.
Sato, Haruo
JNC TN8400 99-064, 22 Pages, 1999/10
Four kinds of diffusion experiments; (1)through-diffusion(T-D) experiments for compaction direction dependency, (2)in-diffusion(I-D) experiments for composition dependency of silica sand in bentonite, (3)I-D experiments for initial bentonite gain size dependency, and (4)I-D experiments for the restoration property of an artificial single fracture in compacted bentonite, were carried out using tritiated water which is a non-sorbing nuclide to evaluate the effect of pore structural factors for eompacted bentonite on diffudion. For(1), effective diffusivities (De) in Na-bentonites, Kunigel-V1 and Kunipia-F were measured for 1.0 and 1.5 Mg m
m . For(2), apparent diffusivities (Da) in Kunigel-V1 were measured for 0.8, 1.4 and 1.8 Mg
. For(2), apparent diffusivities (Da) in Kunigel-V1 were measured for 0.8, 1.4 and 1.8 Mg m
m with silica sand of 30 and 50 wt%. For(3), Da values for 0.8, 1.4 and 1.8 Mg
with silica sand of 30 and 50 wt%. For(3), Da values for 0.8, 1.4 and 1.8 Mg m
m were measured for a granulated Na-bentonite, OT-9607 which grain-size distribution is in a rang between 0.1 and 5 mm. For (4), Da values in Kunigel-V1 which a single fracture was artificially reproduced and was immersed in distilled water for 7 or 28 days for the restoration of the fracture, were measured for 1.8 Mg
were measured for a granulated Na-bentonite, OT-9607 which grain-size distribution is in a rang between 0.1 and 5 mm. For (4), Da values in Kunigel-V1 which a single fracture was artificially reproduced and was immersed in distilled water for 7 or 28 days for the restoration of the fracture, were measured for 1.8 Mg m
m . Although De values in Kunigel-V1 were approximately the same for both compacted directions over the density, De values for perpendicular direction to compacted direction were higher than those for the same direction as compacted direction in Kunipia-F. For composition dependency of silica sand in bentonite, no significant effect of the mixure of silica sand in bentonite on Da was found. For initial bentonite grain size dependency, Da values obtained for OT-960 were approximately the same as those for Kunigel-V1 and no effect of initial grain size of bentonite on diffusion was found. For the restoration property of a single fracture in compacted bentonite, no restoration period dependency on Da was found. Based on this, it may be said that diffusion of nuclides in compacted bentonite, ...
. Although De values in Kunigel-V1 were approximately the same for both compacted directions over the density, De values for perpendicular direction to compacted direction were higher than those for the same direction as compacted direction in Kunipia-F. For composition dependency of silica sand in bentonite, no significant effect of the mixure of silica sand in bentonite on Da was found. For initial bentonite grain size dependency, Da values obtained for OT-960 were approximately the same as those for Kunigel-V1 and no effect of initial grain size of bentonite on diffusion was found. For the restoration property of a single fracture in compacted bentonite, no restoration period dependency on Da was found. Based on this, it may be said that diffusion of nuclides in compacted bentonite, ...
Sato, Haruo
JNC TN8400 99-060, 12 Pages, 1999/10
Apparent diffusion coefficients(Da) of Cs(Cs ), Ni(Ni
), Ni(Ni ) and Se(SeO
) and Se(SeO
 ) in a Na-bentonite (Kunigel-V1) were measured for a dry density of 1.8 Mg
) in a Na-bentonite (Kunigel-V1) were measured for a dry density of 1.8 Mg m
m with silica sand of 30 wt% at room temperature by in-diffusion method to evaluate the effect of the mixture of silica sand on Da in bentonite. The experiments for Cs and Ni were carried out under aerobic condition, but those for Se which is redox sensitive were carried out in an Ar glove-box (O
with silica sand of 30 wt% at room temperature by in-diffusion method to evaluate the effect of the mixture of silica sand on Da in bentonite. The experiments for Cs and Ni were carried out under aerobic condition, but those for Se which is redox sensitive were carried out in an Ar glove-box (O concentration
concentration  0.1 ppm). Consequently, no significant effect of silica sand mixture to the bentonite on Da values of Cs and Se was found, and the obtained Da values were approximately the same as those in the system without silica sand reported so far. On the other hand, Da values of Ni in bentonite with silica sand were 2 orders of magnitude lower than those in bentonite without silica sand obtained to date. The Da values of Ni reported so far were obtained using stable isotopic tracer and a tracer solution with fairly high Ni concentration compared with concentration used in this study was introduced. Additionally, it is known that distribution coefficient (Kd) of Ni on Na-montmorillonite which is the major constituent clay mineral of Kunigel-V1 decreases with increasing Ni concentration. Based on this, the abrupt decrease in Da values of Ni for bentonite with silica sand is considered to be due to the difference of sorption caused by the difference of Ni concentration in the porewater of bentonite.
0.1 ppm). Consequently, no significant effect of silica sand mixture to the bentonite on Da values of Cs and Se was found, and the obtained Da values were approximately the same as those in the system without silica sand reported so far. On the other hand, Da values of Ni in bentonite with silica sand were 2 orders of magnitude lower than those in bentonite without silica sand obtained to date. The Da values of Ni reported so far were obtained using stable isotopic tracer and a tracer solution with fairly high Ni concentration compared with concentration used in this study was introduced. Additionally, it is known that distribution coefficient (Kd) of Ni on Na-montmorillonite which is the major constituent clay mineral of Kunigel-V1 decreases with increasing Ni concentration. Based on this, the abrupt decrease in Da values of Ni for bentonite with silica sand is considered to be due to the difference of sorption caused by the difference of Ni concentration in the porewater of bentonite.
Sugita, Yutaka; Chijimatsu, Masakazu*; Fujita, Tomoo; Tranduc, P.*
JNC TN8430 99-009, 45 Pages, 1999/06
It is an important part of the near field performance assessment of nuclear waste disposal to evaluate coupled thermo-hydro-mechanical (T-H-M) phenomena, e.g., thermal effects on groundwater flow through rock matrix and water seepage into the buffer material, the generation of swelling pressure of the buffer material, and thermal stresses potentially affecting porosity and fracture apertures of the rock. An in-situ T-H-M experiment named "Engineered Barrier Experiment" was conducted at the Kamaishi Mine, in which the host rock is granodiorite, in order to establish conceptual models of the coupled T-H-M processes and to build confidence in mathematical models and computer codes. This report summarizes the results of the in-situ direct compaction technique to evaluate the appropriate conditions for this technique. The in-situ direct compaction technique is one of the major candidate emplacement techniques for the buffer material. This experiment consisted of the mock-up tests and the in-situ test. The mock-up tests showed the appropriate conditions for the in-situ direct compaction technique. For the in-situ experiment, the manufactured OT-9607 achieved dry density averaged 1.65 g/cm , which matched the demand for the thermo-hydro-mechanical experiment.
, which matched the demand for the thermo-hydro-mechanical experiment.